Antimicrobial Photodynamic Therapy Device with Organ/ Application Specific Attachments
Graphical Executive Summary
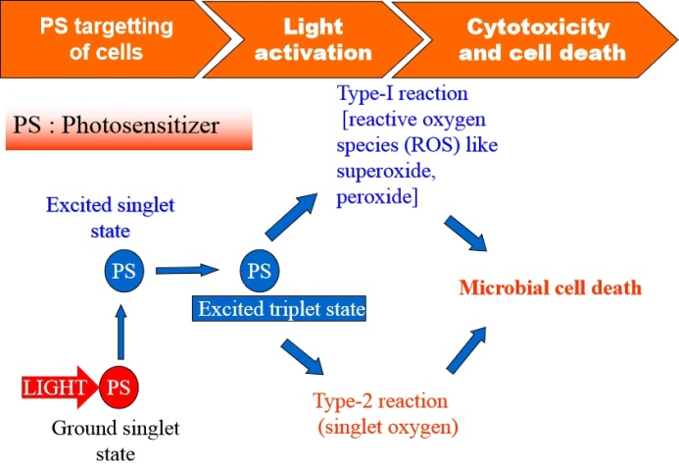
(Mechanism of Antimicrobial Photodynamic Therapy).
Mankind is currently locked in a high-stakes race against drug resistant microbial adversaries that can spawn in a time scale before we can develop new drugs to fight them. Ever since the first antibiotic penicillin was discovered, antibiotic resistance is involved in a cat-and-mouse game with antibiotic development. But, in the last decade the incidences of antibiotic resistance have increased menacingly, propelling world towards a situation of “no action today - no cure tomorrow”. Though new lines of therapeutic strategies have been investigated in the last two decades, resistance in microbes against these investigational strategies has also been reported. With very few strategies in the offing, altogether novel therapeutic interventions are needed urgently to circumvent antibiotic resistance. At RRCAT, we have been actively pursuing studies on antimicrobial photodynamic therapy (APDT), which is an offshoot of photodynamic therapy (PDT) meant for treatment of tumors. APDT involves incubation of light activable drugs called photosensitizers (PS) with microorganisms for a suitable duration followed by exposure to visible light of suitable wavelength. The ensuing photochemical reactions rapidly generate reactive oxygen species which cause damage to multiple biomolecular targets within microbial pathogens. The distinct advantage of APDT is that there is least chance of development of resistance. In the last half decade, our investigations have been focused on APDT mediated inactivation of antibiotic sensitive and resistant bacteria using either cationic PS or cationic peptide conjugated anionic PS (chlorophyll derivative). These investigations have suggested that a suitable APDT window can be optimized that result in ~99 % bacteria load reduction, with concomitant increase in healing response of wounds. In addition to antibacterial applications, we have recently forayed into APDT mediated inactivation of fungi and viruses, notably SARS CoV-2. Propelled by these interesting and encouraging results, we have come up with the idea of developing first-of-its-kind in India “APDT device with organ specific attachments”. These hand-held point of care devices are meant for treatment of infections of external nares (nasal cavity), oral cavity, acne and wound infections in diabetic individuals, subjects undergoing chemo/radiotherapy, immunocompromised human subjects etc., to name a few.

RRCAT and Dr Cure and Care, New Delhi signed a collaborative incubation agreement to develop these technologies. Efficacies of these devices plus our customized PS formulations have been tested against various antibiotic resistant microbes under laboratory settings. Further studies in clinical settings are being carried out.
